What Can DP Do too? | Machine Learning Accelerated Molecular Dynamics Reveals the Ion Diffusion Kinetics in Sulfide Solid-State Electrolytes
Recently, Associate Professor Xu Yang from the School of Science at Shenyang Aerospace University, in cooperation with Professor Fei Du and Professor Yi Zeng from Jilin University and other scholars, conducted an in-depth study on the cubic phase K3SbS4 solid-state electrolyte based on the DeePMD method. The related research results were published in the journal "Chemistry of Materials" under the title "Cl-Doped Cubic K3SbS4 as a Solid-State Electrolyte for K-Ion Batteries with Ultrafast Ionic Conductivity" (DOI: 10.1021/acs.chemmater.4c02575).
Research Background
The development of efficient solid-state electrolytes (SSEs) is crucial for the progress of energy storage technologies, especially in the context of potassium-ion batteries, and is of great significance for improving the safety and stability of batteries. The relatively large radius of potassium ions makes it difficult to transplant many lithium-ion solid-state electrolytes into potassium-ion batteries.
The cubic phase A3MS4 (A = Na, K; M = Sb, P) materials have an open three-dimensional framework structure, which is beneficial for the rapid transmission of ions. However, due to the full occupation of the A site by alkali metals and the effect of electrostatic repulsion, it is difficult for them to diffuse to adjacent A sites, so the unmodified materials usually have a very low ionic conductivity. Complex experimental explorations are often time-consuming and laborious, and the technical obstacles in phase synthesis limit the rapid development of potassium solid-state electrolytes.
Molecular dynamics simulation has become an important tool for understanding the properties and mechanisms of solid-state electrolytes. However, the first-principles molecular dynamics simulation (AIMD) is slow in calculation because it needs to continuously solve the Schrödinger equation, and the atom system that can be simulated usually does not exceed 300 - 500 atoms. Due to insufficient sampling, the results of each simulation for the same system at the same temperature may vary, as has been shown in our previous article.1
This study focuses on the impact of Cl doping on the potassium-ion diffusion rate in the cubic phase K3SbS4 using the DeePMD method. The doped material can achieve an ionic conductivity of 14.8 mS/cm at a temperature of 300 K. Thanks to the relatively fast calculation speed, we also evaluated the impact of sampling time and model size on the reproducibility of simulation results. The results show that a simulation of a 3400-atom system for 500 ps can ensure good reproducibility.
Research Results
Firstly, we used the DP-GEN software for sampling. After multiple cycles such as potential function training, phase space exploration, and single-point energy calculation, we conducted sufficient sampling in the temperature range from 300 K to 1500 K. Subsequently, after a long training of 5 million steps, the potential function we obtained achieved a precision comparable to that of DFT calculations in terms of energy, force, and radial distribution function description, as shown in Figure 1.
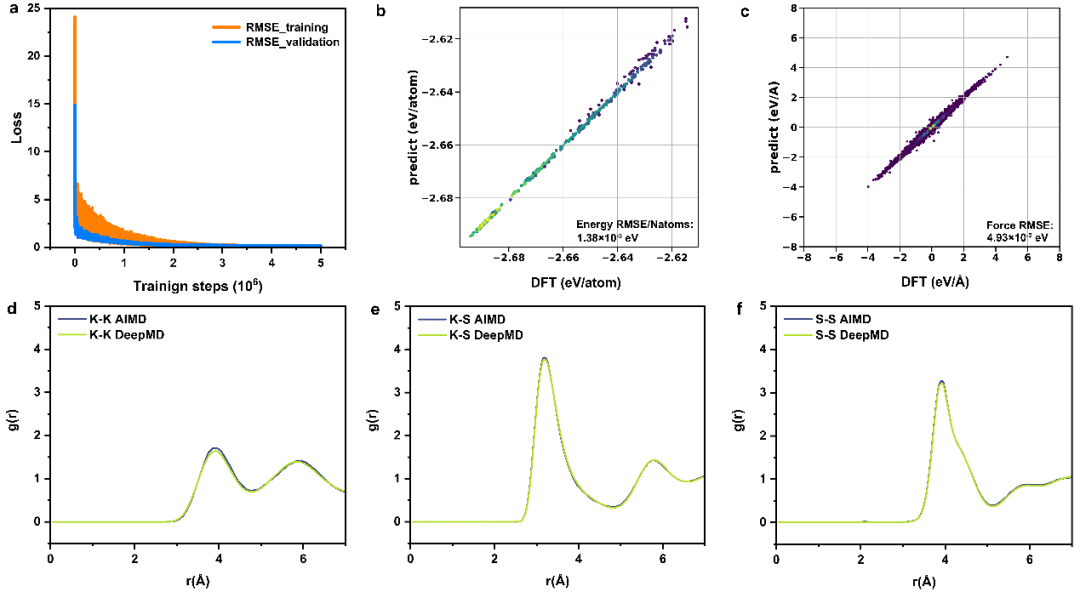
Figure 1: Test on the Training Precision of the Potential Function
We also tested the size of the model and the simulation time. In each set of control experiments, we conducted 5 simulations to calculate the average and standard deviation of the ion diffusion coefficient, as shown in Figure 2. The research shows that using a 6×6×6 supercell (containing 3400 atoms) for a 500 ps simulation can effectively reduce the fluctuation effect caused by insufficient sampling, and the time required for a single simulation is only 1.5 hours.
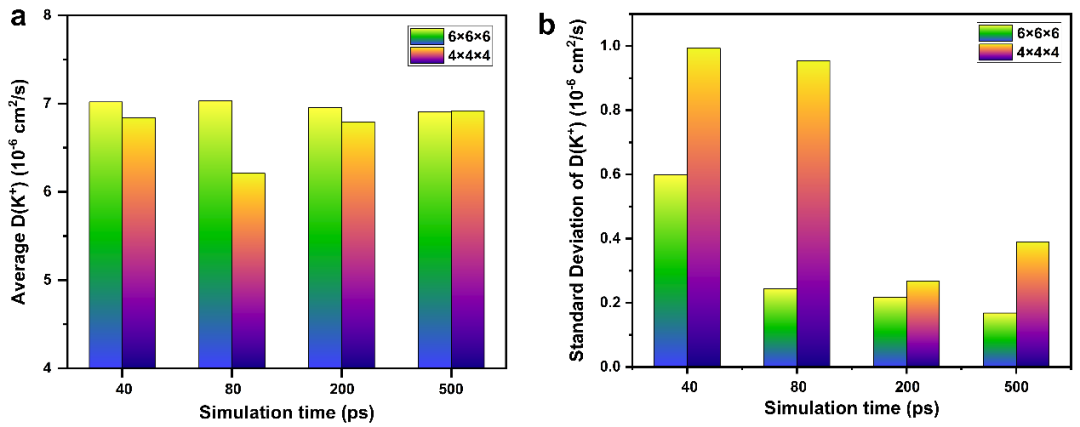
Figure 2: Test on Model Size and Simulation Time
Further, we used DeePMD to conduct a systematic study of the system. The doping of Cl at the S site introduced K vacancies, which promoted the vacancy diffusion of neighboring K positions and thus significantly increased the transmission rate. By analyzing the motion trajectory of potassium ions (see Figure 3), it can be clearly observed that the K ions change from thermal motion near the equilibrium position to diffusion in three-dimensional directions. The trajectory analysis results show that a small amount of doping can increase the ionic conductivity to 14.8 mS/cm, and the corresponding activation energy is only 0.12 eV.
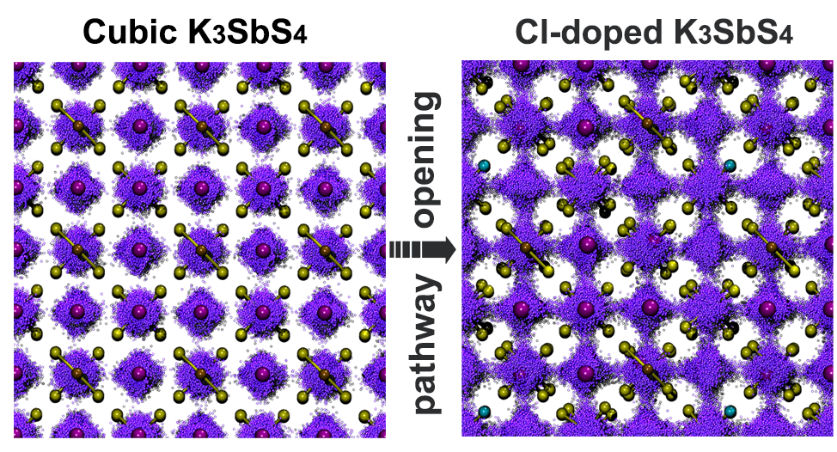
Figure 3: Influence of Doping on the Trajectory of Potassium Ions
Finally, we introduced the Van Hove correlation function.2 It can be seen that for the undoped system (Figure 4a, c), the correlation function parts Gs(t,r) of the same particles at different times and the correlation function parts Gd(t,r) of different particles are relatively stable, which indicates that the particles do not migrate. In contrast, for the doped system, whether it is the disappearance of Gs(t,r) in r = 1 Å or the gradual accumulation of Gd(t,r) with time, both show the typical characteristics of highly correlated jumps of particles.
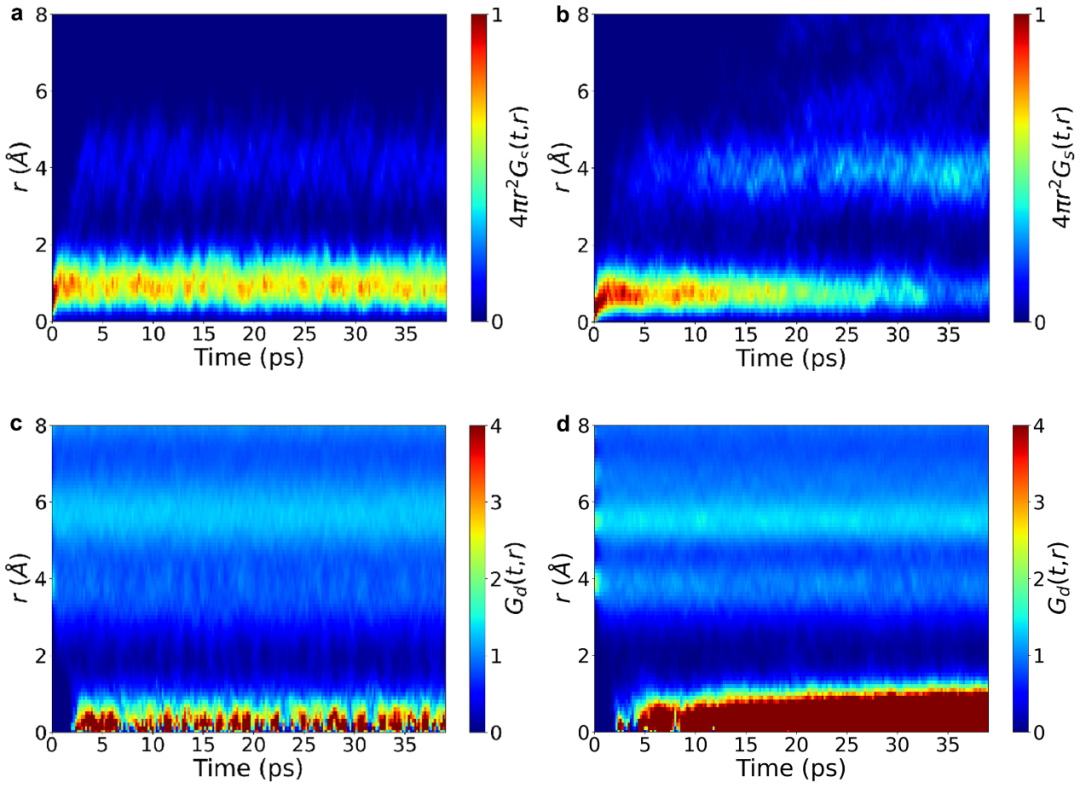
Figure 4: Van Hove Correlation Function of the Undoped and Doped Systems
Summary
We used a neural network to train the potential model of the cube K3SbS4, and systematically studied the impact of model scale and simulation time on quantum fluctuations. On this basis, we analyzed the impact of chlorine doping on the ion diffusion coefficient through the DeePMD method. The research results show that vacancies play a crucial role in the ion diffusion process, and even a small number of vacancies can transform this material from an ion insulator to an efficient ion conductor. The analysis of the Van Hove correlation function further reveals that potassium vacancies significantly increase the jump frequency of potassium ions. At 300 K, the ionic conductivity of this material is as high as 14.8 S/cm, and its diffusion energy barrier is only 0.12 eV. Therefore, the doped cube K3SbS4 is regarded as a promising potassium-ion solid-state electrolyte.
Outlook
The interface study of solid-state electrolytes is currently a hot and difficult topic in computational simulation research. The combination of DeePMD and enhanced sampling will become a powerful tool for studying such complex problems.
References
Zhang, R.; Xu, S.; Wang, L.; Wang, C.; Zhou, Y.; Lü, Z.; Li, W.; Xu, D.; Wang, S.; Yang, X., Theoretical Study on Ion Diffusion Mechanism in W-Doped K3SbS4 as Solid-State Electrolyte for K-Ion Batteries. Inorganic Chemistry 2024, 63 (15), 6743-6751.
Van Hove, L., Correlations in Space and Time and Born Approximation Scattering in Systems of Interacting Partitives. Phys. Rev. 1954, 95 (1), 249-262.