What Can DP Do too? | Science Sub-journal, Understanding More Realistic Solutions, ML Force Field Speeds Up by Six Orders of Magnitude, More Efficiently Representing the Spatiotemporal Relationship of Water Molecules
Water is not only one of the most familiar substances to humans but also a central figure in the long history of physical chemistry. The tetrahedral arrangement and network interactions between its molecules distinguish it from simple liquids.
For a long time, there has been no specific conclusion regarding whether there is a liquid - liquid critical point (LLCP) in water. Besides, researchers' understanding of water, especially when it acts as a solvent, is still incomplete.
To address the problem of technically and reasonably representing the thermodynamic and kinetic properties of water after the introduction of other chemical substances, a team from Seoul National University in South Korea proposed a scheme to examine the spatiotemporal characterization of water using a machine learning force field (MLFF) through deep potential molecular dynamics (DPMD).
This research, titled "Spatiotemporal characterization of water diffusion anomalies in saline solutions using machine learning force field", was published in "Science Advances" on December 11, 2024.
Currently, most water models are unable to fully capture the dynamic behavior of water after the addition of salt. Although classical force fields provide important insights, their simplifications and the omission of dynamic charge effects may distort our understanding of the real behavior of water.
The application advantages of MLFF in fields such as materials science and its processing speed, which is more than six orders of magnitude faster than first - principles methods in systems composed of several hundred atoms, make it stand out among all the options.
DPMD Method
Deep potential MD (DPMD), a popular MLFF model, was used by the team to study the water dynamics in salt solutions.
They compared and analyzed DPMD with other established methods for simulating water and ion dynamics, such as MD with flexible variants of the simple point charge model (SPC/Fw) and the Joung - Cheatham model (JC), etc.
Salt solutions with alkali - loving cations Li and Na exhibit a slowdown in water dynamics as the concentration increases, and this phenomenon has been observed in all the considered models.
Although the quantitative agreement between different models varies, this trend has been observed in many reports. However, from the results, the simulation of pure water differs from the experimental benchmark, which is a common problem with many DFT functionals. Attributed to over - structuring, this may lead to an overestimation of the effect of salt on water dynamics.
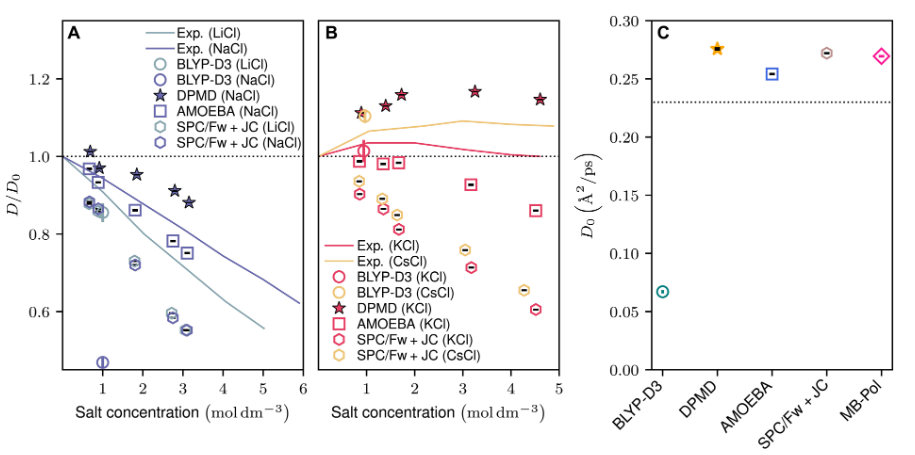
Figure 1: Comparison of the diffusion characteristics of water in salt solutions in various simulation models and experimental benchmarks.
Changing the energy landscape around the ions, especially by changing the energy barrier to release or escape water from the solvation shell, provides a feasible explanation for the observed diffusion acceleration. However, when the assumption of dynamic heterogeneity is no longer applicable, the study of how water dynamics change is an interesting question.
Exploring the reorientation dynamics of water or the entropy effect of salt is an interesting direction for further research. However, the team's attention was drawn to the cooperative motion of water molecules. Recent studies on salt solutions using the mode - coupling theory (MCT) emphasize that the time - dependent correlation dynamics of water can provide a valuable framework.
Although the current models can capture the slowdown of water dynamics after the addition of salt, they do not refute the hypothesis that the dynamic enhancement may result from an accelerated relaxation process.
In contrast, the DPMD model reveals different behaviors. In the case of adding chaotropic salts, its unique ability to accurately reproduce the acceleration of water dynamics highlights the key qualitative difference of DPMD in capturing abnormal water dynamics among various models.
Potential Dynamic Correlation
The test system usually consists of approximately 100 water molecules, and the available analysis time scale is very limited. This situation highlights the key advantage of MLFF as an alternative to first - principles calculations.
It not only establishes itself as a feasible method but also promotes the research team's understanding of water dynamics. This is achieved by effectively managing the computational requirements of large - scale simulations while retaining the valuable insights of first - principles theory.
The team turned their attention to four - point susceptibility. In their research method, it was observed that NaCl produces a higher peak than pure water, while KCl produces a lower peak. This observation may be related to the experimentally observed inhibition of the enhanced water diffusion rate in high - concentration KCl solutions.
The DPMD model also shows that at the lowest concentration, the peak height in the KCl solution is incoherent. These findings indicate that the capabilities of different models have a spectrum to describe the spatiotemporal correlation of water dynamics, depending on the type and concentration of salt.
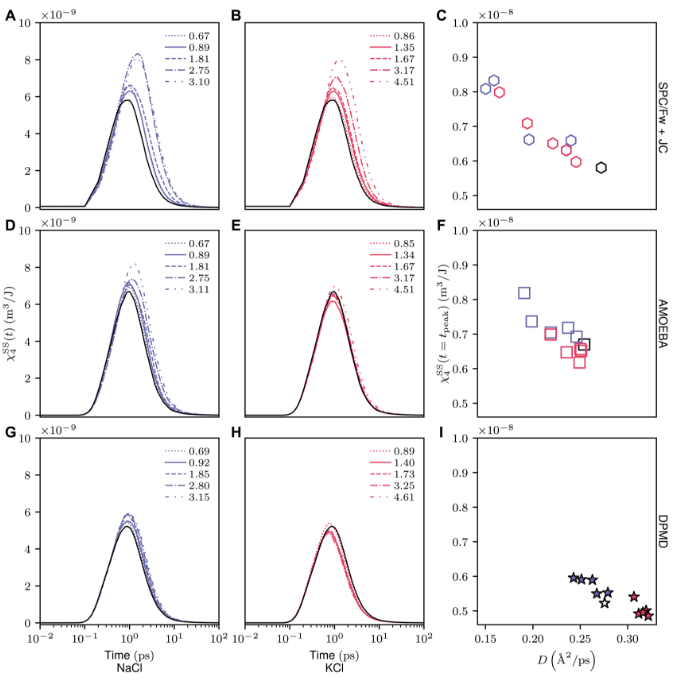
Figure 2: Four - point susceptibility of water at different concentrations in NaCl and KCl solutions analyzed by three computational models.
Considering the abnormal values observed in the relaxation time and correlation length, none of these analyses seem to fully explain the diffusion rate. The team suggests a better understanding of the diffusion rate through a comprehensive perspective of the length scale and time scale.
Exploration and Development
The team said that their work has benefited a lot from the progress in the MLFF field, especially the DPMD model. The research analysis clearly describes the sources of different behaviors in water dynamics by providing a new direction of thinking for the water dynamics in salt solutions and comparing the results obtained with different models.
This comparison not only proves the superiority of DPMD but also opens the door to a more in - depth exploration of the spatiotemporal correlation of water molecules in different environments.
Although the team's analysis did not extensively review the scaling index and thus lacks certain insights into the geometric nature of the correlation. But the exploration in this regard may bring valuable experience to the detailed study of the dynamic structure of water molecules and can also reveal the complex relationship between the structural and dynamic aspects of the collective behavior of water.
Another research direction in the future lies in the field of water reorientation dynamics. By extending the team's analysis to include orientation four - point susceptibility, it is possible to gain a deeper understanding of the collective reorientation of water molecules.
Other results of this study indicate that when the system approaches a deeply supercooled state, there may be an intricate interaction between the correlation length and the relaxation time. This may also reveal the dynamic mechanism implied by the collective behavior of water molecules.
Original link: https://www.science.org/doi/10.1126/sciadv.adp9662